- Industry Solutions
Industry Solutions
From reshaping the quote to cash process, to transforming engagement with channels partners, to achieving excellence in global product launch, Model N enables digital reinvention with industry-specific solutions that maximize revenue.
- Right block
- Industry Solutions
Industry Solutions
From reshaping the quote to cash process, to transforming engagement with channels partners, to achieving excellence in global product launch, Model N enables digital reinvention with industry-specific solutions that maximize revenue.
- Right block
- Products
Our Products
Model N delivers a platform for business reinvention, empowering companies to maximize revenue as they transform Sales, Marketing, Channels, Finance, and Legal processes.
- Right block
- Services & Support
Services & Support
To ensure you get the most out of your Model N investment Model N provides a complete set of professional Customer Success, Services and Support offerings designed to further your business and IT success.
- Right block
- Resources
- Resources
2025 State of Revenue Report
How do leaders plan to optimize revenue in 2025?
Eighty-seven percent of business leaders state that their company is focusing its innovation plans on automating revenue management operations. Learn how you can create smarter, more efficient revenue processes.
- Right block
- Resources
- Company
Company
Model N supports the complex business needs of the world’s leading brands in pharmaceutical, medical device, high tech, manufacturing and semiconductors across more than 120 countries, including Pfizer, AstraZeneca, Sanofi, Gilead, Abbott, AMD, Micron, Seagate, STMicroelectronics, NXP, Sesotec, and Southern States.
- Right block
Contact Sales

 Whitepaper
Whitepaper


 case-studies
case-studies
.
- Products
Our Products
Model N delivers a platform for business reinvention, empowering companies to maximize revenue as they transform Sales, Marketing, Channels, Finance, and Legal processes.
- Right block
- Services & Support
Services & Support
To ensure you get the most out of your Model N investment Model N provides a complete set of professional Customer Success, Services and Support offerings designed to further your business and IT success.
- Right block
- Resources
- Resources
2025 State of Revenue Report
How do leaders plan to optimize revenue in 2025?
Eighty-seven percent of business leaders state that their company is focusing its innovation plans on automating revenue management operations. Learn how you can create smarter, more efficient revenue processes.
- Right block
- Resources
- Company
Company
Model N supports the complex business needs of the world’s leading brands in pharmaceutical, medical device, high tech, manufacturing and semiconductors across more than 120 countries, including Pfizer, AstraZeneca, Sanofi, Gilead, Abbott, AMD, Micron, Seagate, STMicroelectronics, NXP, Sesotec, and Southern States.
- Right block
- Industry Solutions
Industry Solutions
From reshaping the quote to cash process, to transforming engagement with channels partners, to achieving excellence in global product launch, Model N enables digital reinvention with industry-specific solutions that maximize revenue.
- Right block
- Products
Our Products
Model N delivers a platform for business reinvention, empowering companies to maximize revenue as they transform Sales, Marketing, Channels, Finance, and Legal processes.
- Right block
- Services & Support
Services & Support
To ensure you get the most out of your Model N investment Model N provides a complete set of professional Customer Success, Services and Support offerings designed to further your business and IT success.
- Right block
- Resources
- Resources
2025 State of Revenue Report
How do leaders plan to optimize revenue in 2025?
Eighty-seven percent of business leaders state that their company is focusing its innovation plans on automating revenue management operations. Learn how you can create smarter, more efficient revenue processes.
- Right block
- Resources
- Company
Company
Model N supports the complex business needs of the world’s leading brands in pharmaceutical, medical device, high tech, manufacturing and semiconductors across more than 120 countries, including Pfizer, AstraZeneca, Sanofi, Gilead, Abbott, AMD, Micron, Seagate, STMicroelectronics, NXP, Sesotec, and Southern States.
- Right block
Quick Links


Fill out the form to download this whitepaper
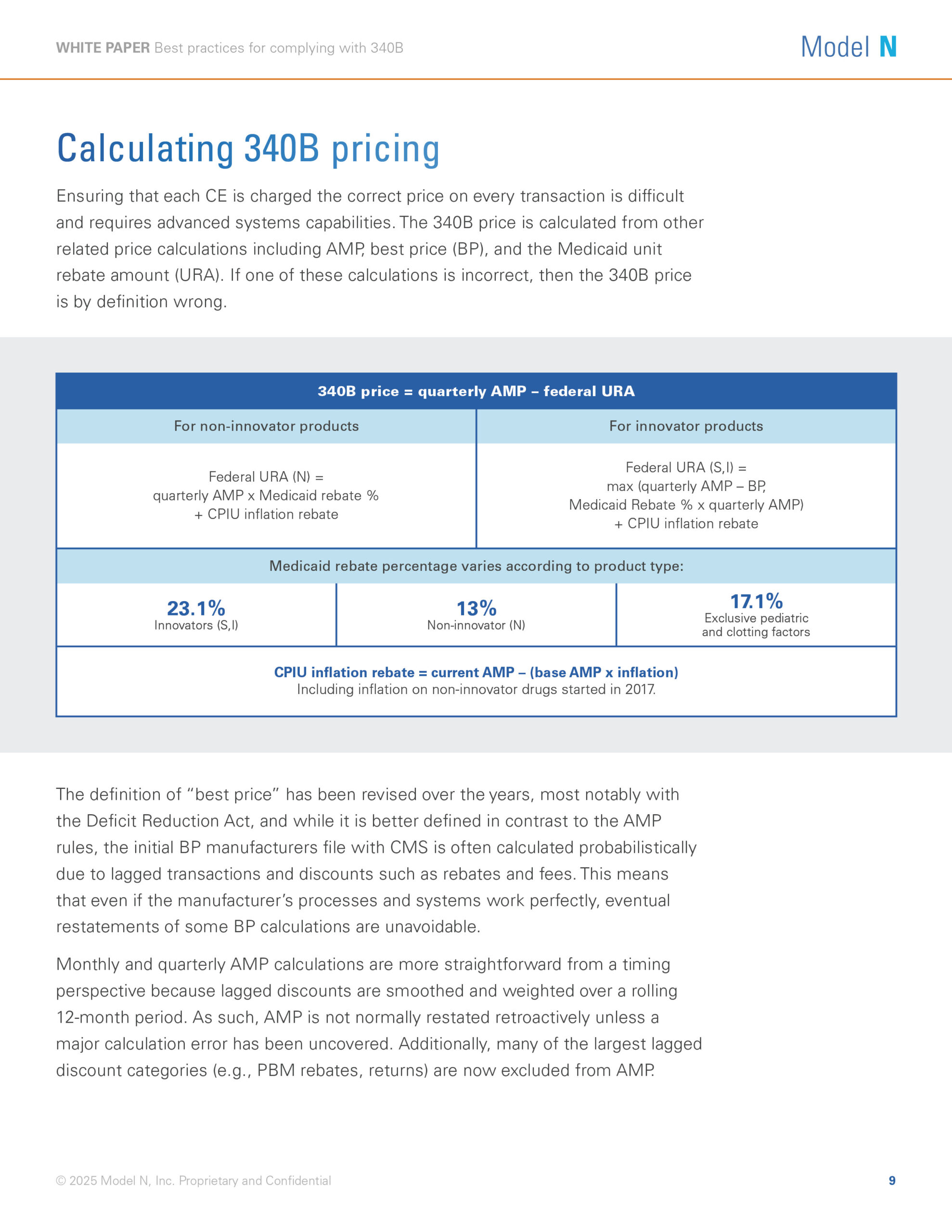
The Public Health Service Act 340B Drug Pricing Program enables providers that serve vulnerable patient populations to access outpatient drugs at significantly lower costs, which helps improve overall patient care. But for pharmaceutical manufacturers, program management can be extremely challenging.


Changes to the 340B program have led to an increase in the number of covered entities and contract pharmacies, as well as more stringent financial and legal implications for price errors.
For pharmaceutical manufacturers that participate in the 340B program, effective management of pricing, rebates, and chargebacks is crucial to protecting revenue streams and ensuring compliance. In this white paper, you’ll learn how you can improve your chargeback process, generate an audit trail, and ensure correct 340B pricing.
In this white paper we will:
- Discuss important changes to the 340B Drug Pricing Program.
- Provide best practices and practical approaches for streamlining 340B program management.
- Explain how you can reduce the risk of revenue leakage and noncompliance.
Thank you for your interest in the Whitepaper: Best Practices for Complying with 340B
You might also be interested in



340B Vigilance Case Study
Pharma manufacturer uses 340B Vigilance to prevent duplicate discounts VIEW case-studiesContact Sales
Thank you for your interest in Model N. Please submit the form and our sales team will contact you within 24 hours.
Our Customers Include:
Thank you for reaching out.
We look forward to responding within one business day.











